Lancet Paper Shows Most Popular Hypertension Drug Isn’t Most Effective, Per OHDSI’s LEGEND Study
Thiazide diuretics demonstrate better effectiveness and cause fewer side effects than ACE inhibitors as first-line antihypertensive drugs, according to a report published Oct. 24 in Lancet.
 These results, which provide additional context to the 2017 guidelines for high blood pressure treatment developed by the American College of Cardiology (ACC) and American Heart Association (AHA), were produced by the Columbia DBMI-centered Observational Health Data Sciences and Informatics (OHDSI) network. The study factored insurance claim data and electronic health records from 4.9 million patients across nine observational databases, making it the most comprehensive one ever on first-line antihypertensives.
These results, which provide additional context to the 2017 guidelines for high blood pressure treatment developed by the American College of Cardiology (ACC) and American Heart Association (AHA), were produced by the Columbia DBMI-centered Observational Health Data Sciences and Informatics (OHDSI) network. The study factored insurance claim data and electronic health records from 4.9 million patients across nine observational databases, making it the most comprehensive one ever on first-line antihypertensives.
Columbia researchers George Hripcsak, MD, MS, and Patrick Ryan, PhD collaborated on the Lancet paper “Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis” as part of the network’s ongoing Large-Scale Evidence Generation and Evaluation across a Network of Databases (LEGEND) project, which applies high-level analytics to perform observational research on hundreds of millions of patient records within OHDSI’s international database network.
Columbia researchers believe LEGEND will continue to significantly enhance how real-world evidence is used to study important healthcare questions that impact millions of patients worldwide.
First-Line Thiazide Diuretic Users Experience 15% Fewer Adverse Cardiovascular Outcomes Than ACE Inhibitor Users
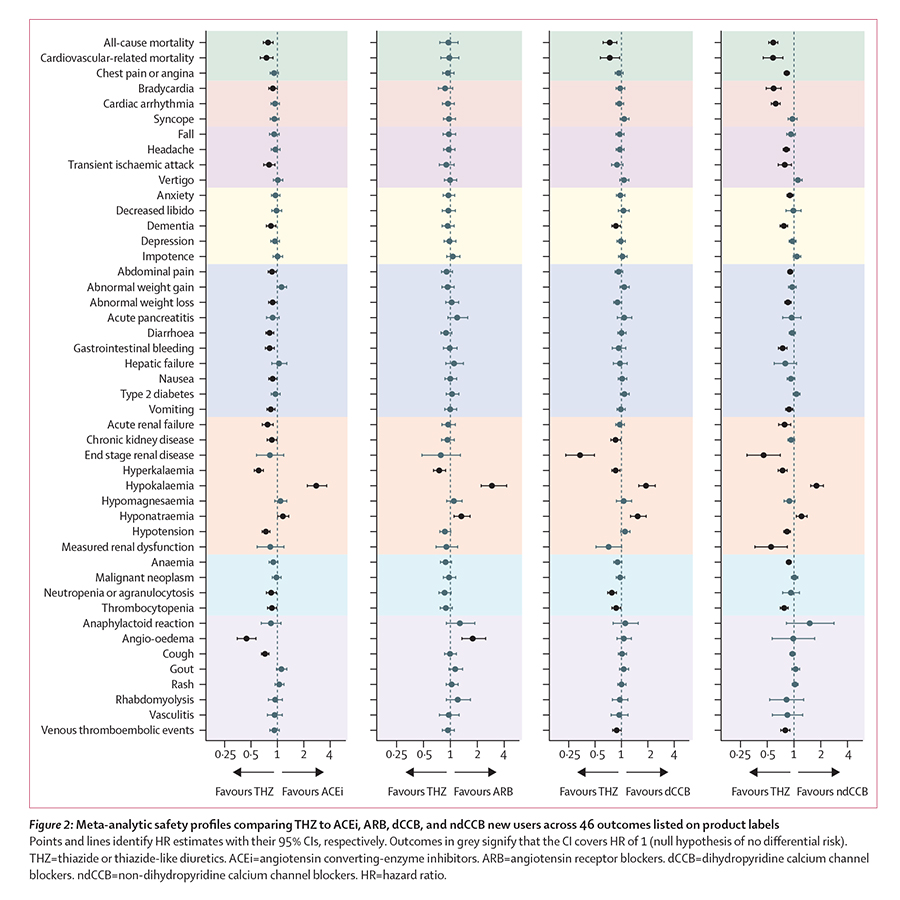
The 2017 ACC/AHA guidelines on antihypertensives recommend initiating hypertension (high blood pressure) treatment with prescription medications from any of five drug classes, including both thiazides and ACE inhibitors. Within the LEGEND project, ACE inhibitors produced both worse cardiovascular outcomes and worse side effects than thiazides.
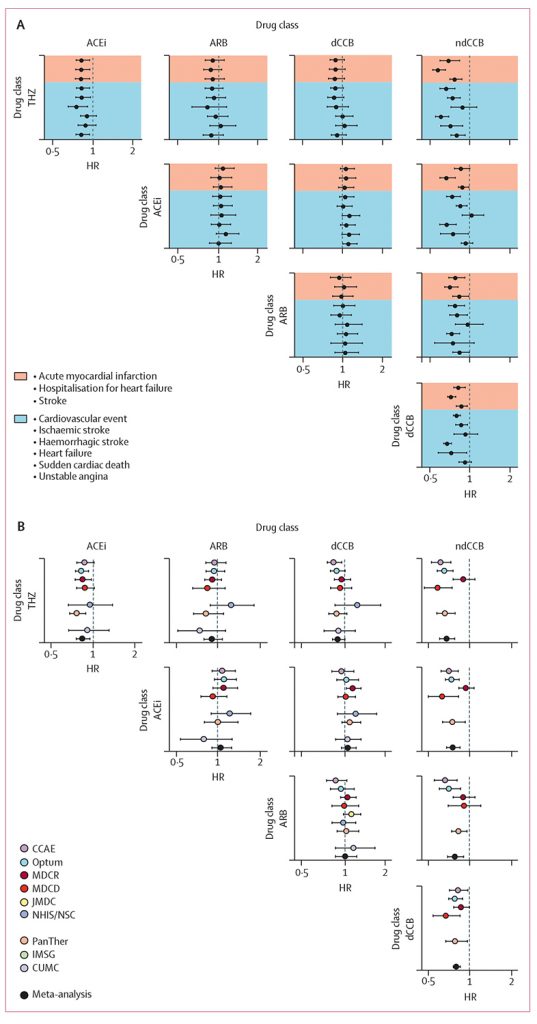
First-line thiazide new-users experienced three major medical outcomes (heart attack, hospitalization for heart failure, and stroke) at an approximate 15% lower event rate than those who began treatment with an ACE inhibitor. Furthermore, among potential side effects associated with first-line hypertensive drugs, ACE inhibitor new-users experienced a higher rate of 19 potential side effects — and a lower rate of 2 — than thiazide diuretic new-users.
In spite of these differences, the majority of patients from this study who initiated treatment were prescribed ACE inhibitors (48%) over thiazides (17%); the results, however, indicate that over 3,100 major cardiovascular events could potentially have been avoided had those approximately 2.4 million ACE inhibitor new-users chosen a thiazide diuretic instead.
Filling The Evidence Gaps
“Patients may not realize that we don’t know all the side effects and comparative efficiency of most drugs,” said Hripcsak, the Vivian Beaumont Allen Professor and Chair of Columbia University’s Department of Biomedical Informatics. “LEGEND was developed by the OHDSI collaborative to do clinical research that fills in evidence gaps left by randomized controlled trials and gives doctors and patients the information they need to support the best care.”
“By testing many drugs and many clinical conditions simultaneously, we are able to comprehensively answer important patient questions, including those relating to side effects and cardiovascular event risk,” Hripcsak added. “OHDSI uses the most extensive series of tests and checks to make sure that its conclusions are justified according to the evidence in the databases.”
The paper also reported that non-dihydropyridine calcium channel blockers proved inferior to the four other first-line antihypertensive drug classes recommended in the 2017 guidelines; other classes included are angiotensin receptor blockers and dihydropyridine calcium channel blockers.
A LEGEND-ary Approach To Observational Science
“LEGEND represents a new way of doing research,” Hripcsak said. “It is one that is highly productive, answering many questions at once, yet is also uniquely careful not to make false claims.”
The LEGEND Hypertension project used state-of-the-art causal methods to address both observed confounding and residual bias. Covering patients from July 1996 to March 2018, the study filled in evidence gaps that were unavailable for the 2017 ACC/AHA guidelines. The RCTs from those guidelines factored approximately 31,000 users of either thiazide diuretics or ACE inhibitors, far fewer than the approximately 3.2 million new-users available in the LEGEND project.
“LEGEND is a novel approach that could transform the way we use real-world evidence in healthcare,” said Ryan, the senior author and an adjunct assistant DBMI professor. “Rather than inefficiently conducting bespoke analyses one-question-one-method-one-database-at-a-time, leaving us vulnerable to various threats to scientific validity, LEGEND provides a systematic framework that can reproducibly generate evidence by applying advanced analytics across a network of disparate databases for a wide array of exposures and outcomes.”
“Not only does LEGEND offer a path to scale to the real needs of the healthcare community,” Ryan added. “it also provides the complementary diagnostics to help us understand how much we can trust the evidence we’ve produced.”
LEGEND was developed through tools and best practices set by the OHDSI network, a multi-stakeholder, interdisciplinary collaborative to bring out the value of health data through large-scale analytics. OHDSI is an open-science community which, through its central coordinating center at Columbia University, has established an international network of research and observational health databases. OHDSI, which has produced scores of peer-reviewed papers since its 2014 inception, develops open-source analytics tools to generate and disseminate real-world evidence.


