OHDSI: Advancing Global Health Research Through Open Data Collaboration; DBMI Drives Mission As Central Coordinating Center
The Observational Health Data Sciences and Informatics (OHDSI) initiative is a global, collaborative effort aimed at transforming healthcare by harnessing the power of large-scale observational data.
OHDSI (pronounced “Odyssey”) set a mission more than a decade ago to improve health by empowering a community to collaboratively generate real-world evidence that promotes better health decisions and better care. To achieve that goal, OHDSI brings together researchers, clinicians, and data scientists to enable real-world evidence generation and knowledge sharing on an unprecedented scale.

Nearly 500 people joined the 2024 OHDSI Global Symposium (Oct. 22-24)
As the central coordinating center, Columbia University plays a pivotal role in guiding the initiative’s development, supporting its diverse community, and providing the infrastructure necessary for OHDSI’s groundbreaking research collaborations across the globe.
Global Collaboration
 OHDSI has brought together more than 4,200 collaborators across 83 countries to “join the journey” towards generating reproducible, reliable and robust real-world evidence for healthcare. An interdisciplinary community, OHDSI encourages collaborators with diverse expertise — including epidemiologists, informaticians, clinicians, statisticians, developers, and regulators — to research together around data standards, methodological research, open-source development and clinical applications.
OHDSI has brought together more than 4,200 collaborators across 83 countries to “join the journey” towards generating reproducible, reliable and robust real-world evidence for healthcare. An interdisciplinary community, OHDSI encourages collaborators with diverse expertise — including epidemiologists, informaticians, clinicians, statisticians, developers, and regulators — to research together around data standards, methodological research, open-source development and clinical applications.
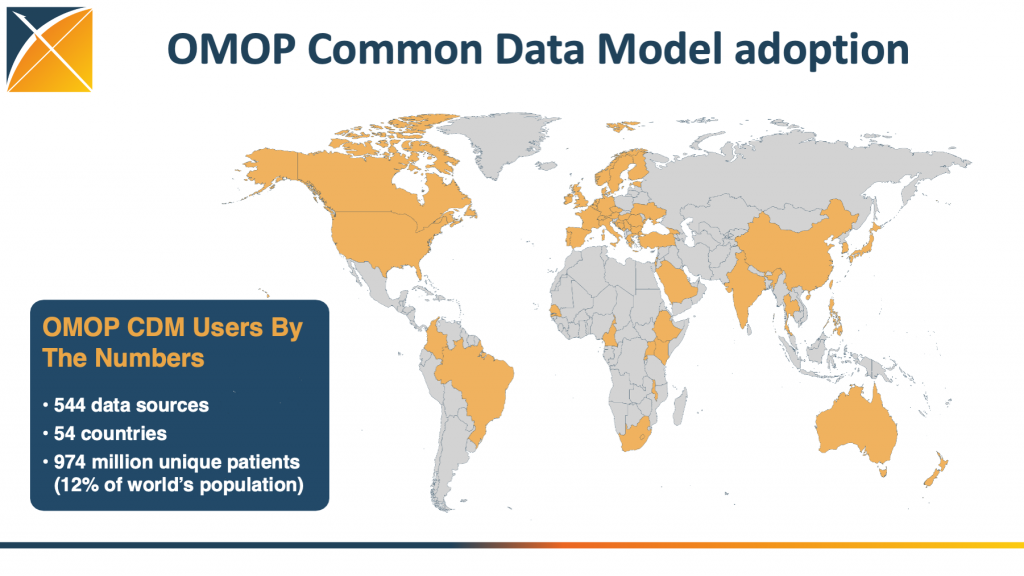
Providing answers requires robust data, and OHDSI has mapped the records of more than 978 million patients from across 54 countries and 544 data sources, to the OMOP Common Data Model, a standardized way of organizing healthcare data from different sources into a common format, making it easier for researchers to analyze and compare large datasets to improve health outcomes.
Global Impact
Through August 2024, the OHDSI community has published more than 730 studies, including several in top clinical journals like JAMA, BMJ, Lancet, JAMA Internal Medicine and JACC. The clinical evidence generated over a decade has informed a range of therapeutic areas, including including hypertension, diabetes, COVID-19, vision care, depression, oncology.
The impact of these studies has been felt around the world, including one that highlighted clinical heterogeneity in treatment pathways among type 2 diabetes mellitus, hypertension, and depression patients. The LEGEND initiative resulted in a Lancet study that impacted clinical guidelines around hypertension treatment. OHDSI generated evidence requested by the European Medicines Agency (EMA) in 2021 that showed there was not an increased risk for clotting due to the AstraZeneca vaccine; several days later, the EMA turned the vaccine back on for more than 700 million Europeans. OHDSI has also done methodological studies, including one on negative controls that was cited as a best practice by the EMA, and it has produced a suite of open-source tools to empower global research, including R packages that have been downloaded more than 800,000 times on CRAN.
Columbia DBMI’s Role
• Providing central shared infrastructure and coordinating community activities to enable community collaborations that advance OHDSI’s mission
• Leading the Steering Workgroup to provide guidance and support to enable the community to collaboratively generate evidence and the scientific work products necessary to generate evidence
• Supporting current OHDSI leaders (workgroups, regional chapters, network studies, etc.) to achieve their objectives by communicating ongoing activities and successful accomplishments, encouraging participation and collaboration throughout the community, and empowering future leaders
• Maintaining infrastructure and providing support to connect collaborators with collaboration opportunities
• Encouraging more visitors to become collaborators
• Providing open access to OHDSI evidence and work products, including:
– Distributing standardized vocabularies
– Supporting open-source software with permissive licenses
– Encouraging open sharing of study design and implementation
– Maintaining open access to study results


